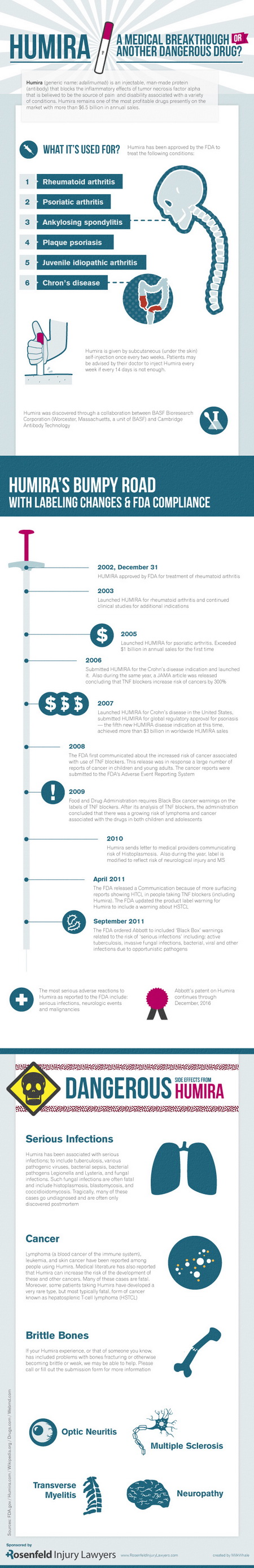
What is Humira – An Infographic
There has been a discussion about whether Humira is a medical breakthrough or another dangerous drug.
Let’s dig more about Humira to find out the cause of the debate. Also, read until the end to see the infographic about it.
What is Humira
Humira is an injectable, man-made protein (antibody) that blocks the inflammatory effects of tumor necrosis factor alpha.
Many believe that tumor necrosis factor-alpha is the source of pain and disability associated with various conditions.
With annual sales of more than $6 billion, Humira is one of the most profitable drugs on the market.
What is Humira Used For?
Humira has received approval from The Food and Drug Administration (FDA) to treat the following conditions, such as:
- Rheumatoid arthritis
- Psoriatic arthritis
- Ankylosing spondylitis
- Plaque psoriasis
- Juvenile idiopathic arthritis
- Crohn’s disease
Patients should do the self-injection of Humira once every two weeks.
Moreover, they must receive medical advice from their doctor to inject Humira every week if once every two weeks is not enough.
Humira’s Bumpy Road
Humira experienced a bumpy road with labeling changes and getting FDA compliance.
Here’s what to point out from Humira’s development journey.
2002
Humira got approval from the FDA for the treatment of rheumatoid arthritis.
2003
Humira was launched for rheumatoid arthritis and continued clinical studies for additional indications.
2005
Humira exceeded $1 billion in annual sales for the first time
2007
Humira submitted for global regulatory approval for psoriasis. Also, Humira generated more than $3 billion in worldwide sales.
2009
The FDA required Black Box cancer warnings on the labels of TNF blockers. The administration concluded that there was a growing risk of lymphoma and cancer associated with the drugs in both children and adolescents.
2010
Humira sent a letter to medical providers communicating the risk of Histoplasma. Also, it modified the label to reflect the risk of neurological injury and MS.
April 2011
The FDA released a communication because of more reports showing HSTCL in people taking TNF blockers, including Humira. The FDA also updated the product label warning for Humira to include a warning about HSTCL.
September 2011
The FDA ordered Abbott to include Black Box warnings related to the risk of serious infections, including active tuberculosis, invasive fungal infections, bacterial, viral, and other infections due to opportunistic pathogens.
Abbot’s patent on Humira continued through December 2016.
Dangerous Side Effects from Humira
Some of the dangerous side effects of Humira injections are:
- Serious infections
- Cancer
- Brittle bones
- Optic neuritis
- Transverse myelitis
- Multiple sclerosis
- Neuropathy
Conclusion
Injecting Humira in patients is still possible after a thorough medical checkup and consultation with the doctor.
Side effects may appear differently for each patient. They should consult their doctor immediately when side effects start to appear.
Learn the following infographic to discover more about Humira.